Vaccine development for COVID-19 and seasonal flu is, in many respects, a high-stakes game of cat and mouse. Because these viruses mutate rapidly and vaccines target specific strains, protection fades quickly as new variants emerge. Maintaining population immunity therefore requires repeated reformulation and revaccination.
In this context, Generation Gold Standard—a U.S. Department of Health and Human Services (HHS) and National Institutes of Health (NIH) initiative to develop universal flu and coronavirus vaccines—marks a welcome pivot toward longer-lasting protection. Universal vaccines aim to provide immunity against many or all strains of a disease—ideally even those that don’t exist yet. This could resolve major inefficiencies in the existing booster-vaccine paradigm. The administration deserves credit for recognizing the strategic value of investing in universal vaccines.
Yet the initiative’s potential risks being undercut by a narrow bet: all current investments support vaccine candidates using whole-inactivated virus technology. While this technology has notable strengths, it is just one of many options, each with different pros and cons.
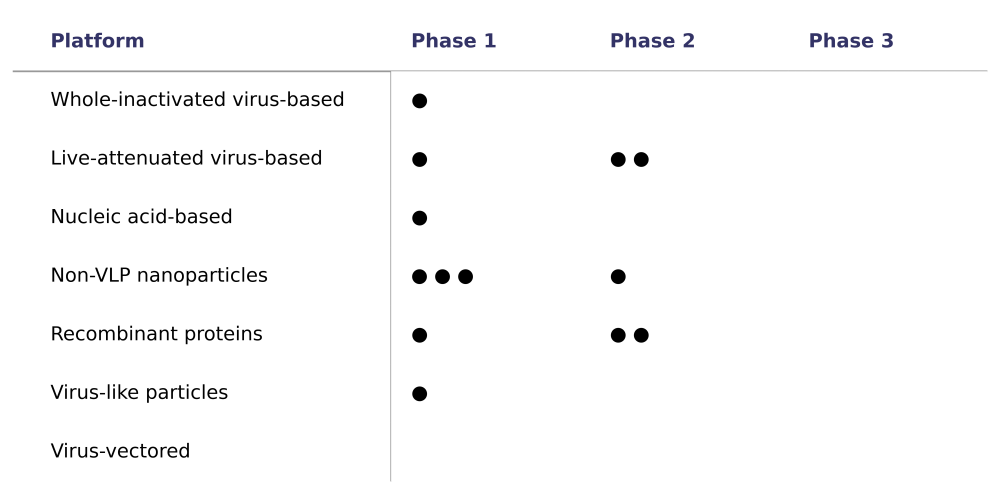
Table 1. Universal flu vaccine candidates across technological platforms and clinical trial stages
Source: Author analysis of data from Universal Influenza Vaccine Technology Landscape.
Note: Each dot represents a universal flu vaccine candidate under active development. The table only includes vaccine candidates described as “universal or broadly protective” and under “active development.” It does not include candidates described as “next-generation influenza vaccines.”
To fully realize the potential of Generation Gold Standard, HHS should expand its investment strategy and fund vaccine candidates across multiple technological platforms. When the stakes are trillions of dollars in social value, investing in a diverse portfolio is the surest way to deliver impact.
A shot at lasting immunity
Despite major technological advances, vaccine development still struggles to keep pace with the rapid evolution of flu and coronaviruses. mRNA-based COVID-19 vaccines take about two to six months from sequencing to manufacture, while egg-based flu vaccines require six to eight months of lead time. Yet new variants of both viruses can become dominant in under a month. Vaccines formulated months in advance may therefore offer limited protection against circulating strains.
Figure 1. Effectiveness of US seasonal influenza vaccines over time

Source: Author analysis of CDC data.
Note: Seasonal flu vaccine effectiveness can vary substantially year to year, and sometimes dramatically underperforms expectations. During the 2014–15 flu season, unexpected antigenic drift caused efficacy to fall to just 6 percent against the predominant variant. Vaccine effectiveness is defined as the proportional reduction in disease risk among vaccinated individuals, compared to unvaccinated individuals, under real-world conditions.
Universal vaccines eliminate the need for constant reformulation. Even more importantly, they enable pre-emptive immunization, protecting populations before infections surge. According to one study, replacing seasonal flu vaccines with a universal alternative could prevent 1.8 million infections, nearly 2000 deaths, and $341 million in medical costs in the U.S. every flu season. Less frequent vaccination may also increase vaccine uptake, particularly if administered intranasally, as one of the NIH’s candidates is targeting.
The biggest payoff, however, may come during the next pandemic. Zoonotic flu and coronavirus outbreaks are not once-in-a-century events: one estimate suggests a COVID-19-scale outbreak could occur every 33 to 50 years, resulting in the equivalent of 2.5 million annual deaths worldwide. Having broad immunity and a proven vaccine in place before a new outbreak begins could sharply reduce mortality and social losses from future pandemics. It is to the administration’s credit that Generation Gold Standard appears to target influenza and coronavirus variants with higher pandemic potential.
One platform is a risky dose
The whole-inactivated virus technology used in candidates supported by Generation Gold Standard is a worthwhile bet: it’s a proven approach, and its relatively modest cold-chain requirements could simplify deployment in low-resource settings.
Nonetheless, whole-inactivated virus vaccines, like all technologies, carry unique challenges and risks. Several vaccine experts have commented on the technology’s potential to trigger strong adverse reactions, which could complicate regulatory approval or dampen public confidence. Whole-inactivated virus vaccine production also introduces biosafety risks, as it requires cultivating large amounts of live virus, and may further tie us to egg-based vaccine manufacturing, a process that is poorly suited to the speed, adaptability, and supply chain robustness desired in a pandemic.
In general, history suggests we should not expect to identify the winning approach in advance. Before 2020, mRNA vaccines were viewed as speculative by many, yet they became the first COVID-19 vaccines authorized in the U.S., and ultimately accounted for more than 90 percent of American COVID-19 vaccinations.
Generation Gold Standard’s support for whole-inactivated virus vaccines makes sense as part of a larger portfolio. However, betting on a single technology unnecessarily narrows our odds. Of the 13 universal flu vaccine candidates currently under development, only one relies on whole-inactivated virus technology (see Table 1). The remaining 11 span at least five different technological platforms, including recombinant proteins, mRNA, and nanoparticle-based approaches. With no candidates beyond phase 2 clinical trials, their prospects remain uncertain and contingent upon further results.
Portfolio thinking for pandemic preparedness
To strengthen Generation Gold Standard, the U.S. should build on its current investment by adopting a portfolio approach that supports multiple promising vaccine candidates across diverse technological platforms. This does not mean displacing existing support for whole-inactivated virus vaccines, but rather complementing it with broader bets.
Investing across a wider range of vaccine platforms, such as recombinant, viral vector, or mRNA platforms, raises the odds of technical success and reduces the risk of correlated failure. It could also help build a safer, more agile, and more scalable flu vaccine supply chain in line with the U.S. government’s National Influenza Vaccine Modernization Strategy.
Broader investments could take the form of competitive grants to a range of vaccine candidates. They could also be paired with pull funding that rewards success on outcomes such as safety, universality, and real-world effectiveness. As White House Science Advisor Michael Kratsios has noted, outcome-based funding can multiply the impact of federal research dollars, as it directs resources toward what works, cutting waste and accelerating progress. By linking rewards to technical milestones and real-world uptake, pull funding can also foster greater consumer choice while incentivizing strong performance on key traits like durability and universality. With many candidates, limited commercial incentives, and large returns to fast innovation, universal vaccines are a textbook case for pull funding.
Figure 2. Examples of “push” and “pull” funding

Note: Push funding involves paying innovators for upfront costs, while pull funding conditions payments on outcomes.
Universal vaccines offer extraordinary benefits, but success in biomedical innovation is rarely predictable. Generation Gold Standard is a great start, but focusing on one technology could slow us down when speed and flexibility matter most. The best way to back bold innovation is to build a portfolio.
This blog is cross-posted on the Center for Global Development website.
Thumbnail image by: Mikhailov Studio/ Adobe Stock